Benzyl alcohol is also known as Aromatic Alcohol with the chemical formula C6H5CH2OH. The IUPAC name of this compound is phenyl methanol. At room temperature, benzyl alcohol exists as a colourless liquid that has a mildly aromatic smell. When this aromatic alcohol is deprotonated, the resulting anion is called a benzylate.
This compound is not very soluble in water. However, it forms miscible mixtures with diethyl ether and other alcohols. Many plants are known to naturally produce C6H5CH2OH. The essential oils extracted from jasmine, ylang-ylang, and hyacinth contain some amount of benzyl alcohol.
· Properties of Benzyl Alcohol
· Preparations of Benzyl Alcohol
· FAQs
This compound consists of a hydroxyl group attached to a methyl group, which is in turn attached to an aromatic ring. The structure of a C6H5CH2OH molecule is illustrated below.
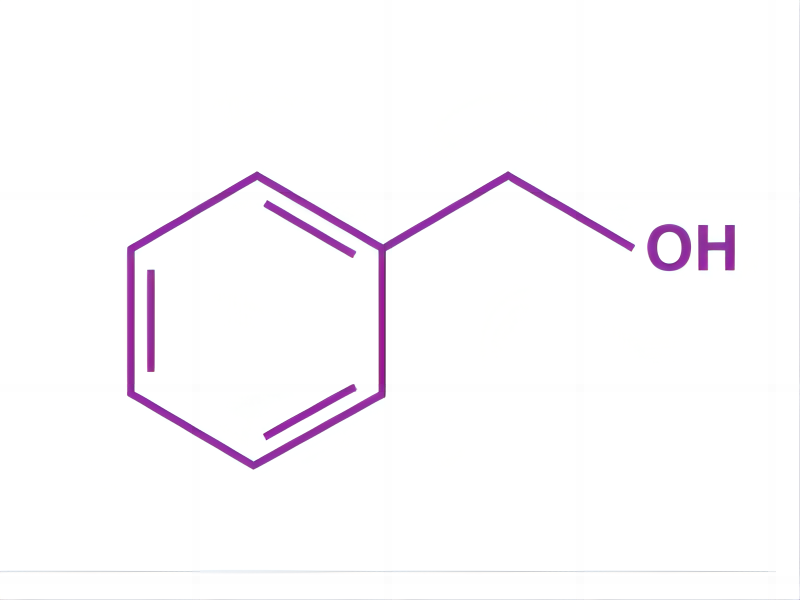
The pi electrons in the benzene ring are delocalized due to resonance. Essentially, the structure of a benzyl alcohol molecule is that of a toluene molecule in which one of the hydrogen atoms has been replaced by a hydroxyl group.
· Under standard conditions, benzyl alcohol is a colourless, slightly aromatic liquid.
· Its solubility in water corresponds to 3.5g/100mL at 20oC and 4.29g/100mL at 25o
· This compound is soluble in several organic solvents such as benzene, methanol, acetone, and ether.
· The reaction between carboxylic acids and benzyl alcohol leads to the formation of esters.
· This compound undergoes a Ritter reaction with acrylonitrile to yield N-benzyl acrylamide.
· When deprotonated, C6H5CH2OH yields a benzylate anion.
The use of sodium hydroxide in the hydrolysis of benzyl chloride yields benzyl alcohol and sodium chloride as the products. The chemical equation for this reaction is given by:
NaOH + C6H5CH2Cl → NaCl + C6H5CH2OH
An alternate method of preparing this compound involves the Grignard reaction between formaldehyde (H-CHO) and phenylmagnesium bromide (Ph-Mg-Br).
This compound is widely used as a solvent for epoxy resin coatings, inks, and paints. Some other applications of benzyl alcohol are listed below.
· C6H5CH2OH is a precursor to several esters.
· A solution of benzyl alcohol with a concentration of 10% can be used as a local anaesthetic and also as an antimicrobial agent.
· This compound is a component of the fluid mixtures used in electronic cigarettes (it enhances the flavour).
· Benzyl alcohol can serve as a dielectric solvent for the reconfiguration of some nanowires via dielectrophoresis.
· 5% solutions of this compound can be used to treat head lice.
· It is used in the manufacture of soaps, shampoos, and skin lotions because of its antifungal and antibacterial properties.
Q1
Benzyl alcohol is a prescription topical (for the skin) medication used as an anti-parasite medication. Benzyl alcohol topical is used to treat head lice in people between the ages of 6 months and 60 years old. Benzyl alcohol topical is used for treating head lice only. It will not treat lice on other body areas.
Q2
In intravenous liquids which are drawn more than once from the same bottle or container, benzyl alcohol is most commonly present. Once the seal of the jar has been breached, its purpose is to slow bacterial growth.
Q3
Benzyl alcohol with the C6H5CH2OH formula is an aromatic alcohol. The benzyl group is also abbreviated to “Bn” (not to be confused with “Bz” used for benzoyl), so BnOH is referred to as benzyl alcohol. Benzyl alcohol is a colourless liquid with a good floral fragrance that is mild.
Q4
Benzyl alcohol is a colourless liquid used as a bacteriostatic and topical local anaesthetic in injection solutions. Isopropyl alcohol is a clear, volatile, colourless liquid used as an antiseptic solvent and disinfectant, commonly known as isopropanol.
Q5
The family of organic compounds known as benzyl alcohols contains benzyl alcohol, also known as alpha-toluenol or benzene methanol. Chemical compounds forming the substructure of phenyl methanol are these. Benzyl alcohol is a (based on its pKa) highly mild simple (essentially neutral) compound.